IndoVac Can Be Used by the Elderly for the Second Booster of the Covid-19 Vaccine
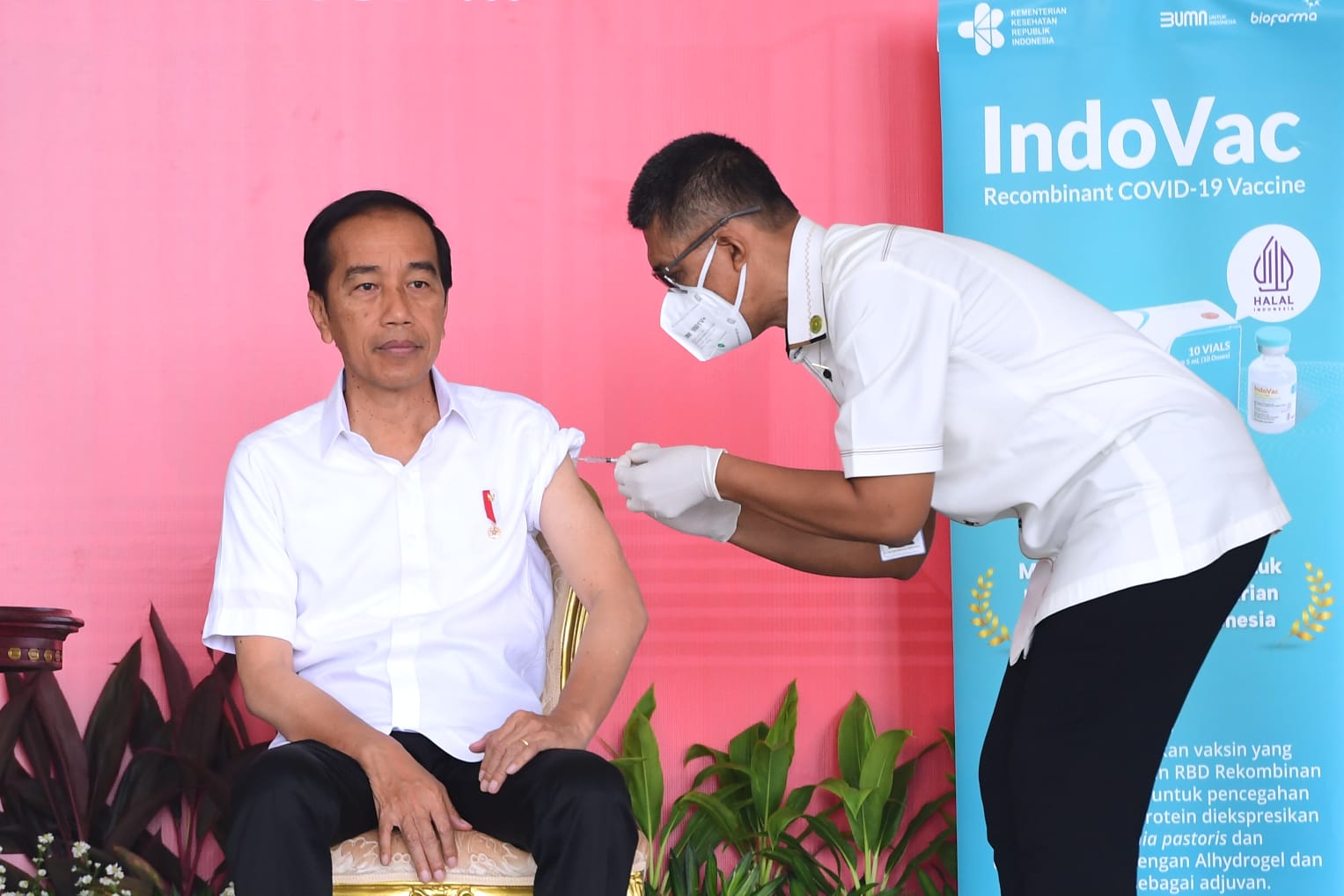
Indonesia President Joko Widodo was the first to receive the IndoVac vaccine, which Bio Farma made.
(Bogor 23/11) The good news for Indonesian people who are included in the elderly group (over 60 years), can already get the second booster vaccine of the Covid-19 vaccine using IndoVac. This is based on Circular Letter Number HK.02.02/C/5565/2022 concerning the 2nd Booster Dose of COVID-19 Vaccination for the Elderly Group which was issued on November 22, 2022, from the Indonesian Ministry of Health.
This activity was also attended by the President of the Republic of Indonesia Joko Widodo, Minister of State Secretary Pratikno, Minister of Health Budi Gunadi Sadikin, Minister of BUMN Erick Thohir, Minister of Public Works and Public Housing Basuki Hadimuljono, Main Director of Bio Farma Honesti Basyir.
The first injection of the IndoVac booster for the elderly was carried out at the Bogor Presidential Palace on November 24 2022, and Joko Widodo was the first to receive the IndoVac vaccine, which was made by Bio Farma. After the injection, in his remarks, Joko Widodo said that Indonesia had injected 205 million doses, for the second dose there were 172 doses, and for the first booster vaccine 66 million doses had been injected and for the second booster, there were still 730 thousand doses of vaccine.
"Why do we need a booster, so that our immunity is maintained and can stop the transmission of Covid. The most important. The vaccine used in this second booster is the indovac vaccine, a 100% domestically made product," said Joko Widodo.
Meanwhile, Budi Gunadi Sadikin said booster vaccination is very important considering that COVID-19 cases in hospitals are increasing For the cases that died, in this last wave 84 percent had not been 'boostered', so for the public please remind them to quickly 'boostered', said Budi Gunadi.
Bio Farma will support the administration of this second booster vaccine for the elderly, by producing at least five million doses of the IndoVac vaccine by the end of 2022, this was conveyed by the Main Director of Bio Farma Honesti Basyir when the second booster injection activity was in progress.
"We will prepare IndoVac vaccines for boosters for the elderly group. The production process has been running since the EUA was issued. for the primary dose at the end of September 2022, at least, we will produce as many as five million doses, until the end of 2022," Honesti said.
Honesti continued that IndoVac could boost the coverage of booster vaccine use, which only reached 36%, "We hope with the issuance of this Circular Letter from the Ministry of Health, the IndoVac vaccine, can help tar achievement get from the Covid-19 booster vaccine in Indonesia, especially for the elderly," he continued.
Giving this booster needs to be done, to provide additional protection for the elderly group who is quite vulnerable to the Covid-19 virus attack, and most importantly to reduce the severity and even death from COVID-19. The second booster dose of vaccination for the elderly can be done at a health care facility (Fasyankes or at a Covid-19 vaccination service).
Before getting the EUA Booster, the Indovac vaccine which was inaugurated by the President of the Republic of Indonesia Joko Widodo on October 13, 2022, had first received the EUA for the primary dose, at the end of September 2022, and at almost the same time, IndoVac had officially received a Fatwa and Decision Halal from the Indonesian Ulema Council (MUI), thus the IndoVac vaccine meets the safe and halal criteria.
Based on the results of the EUA booster from the POM Agency, the IndoVac vaccine is given at intervals of at least six months after the administration of the primary dose of the vaccine, and can be used for those who have received the Sinovac vaccine for the primary dose administration. The IndoVac vaccine will be given in a full dose or as much as 0.5 ml.
Giving this booster needs to be done, considering that on average people who have received the 2nd dose of vaccine, it has been more than six months since the second dose, so there is a potential for our body's immunity to decrease against the Covid-19 virus attack. Thus, the administration of the Covid-19 vaccine booster aims to increase antibodies against the Covid-19 virus for people who have received the full primary dose of the Covid-19 vaccine.
In parallel, a vaccine containing IndoVac Domestic Component Level (TKDN) of 90.3% which is the result of a collaboration between Bio Farma and Baylor College of Medicine from the US, is conducting clinical trials of the IndoVac Covid-19 vaccine for children on October 9, 2022, involving 620 children, in collaboration with Andalas University Medical Faculty (UNAND) Padang. Apart from FK UNAND, the clinical trial will involve as many as 1050 subjects with an age range between 7-17 years, in collaboration with the Faculty of Medicine, University of Indonesia (FK UI) Faculty of Medicine, Gadjah Mada University (FK UGM), for the target of issuing EUA for children Children will be released in early December 2022. (ed)
 ID
ID
 EN
EN




